Using Laboratory Diagnostics to investigate Bovine Respiratory Disease (BRD) in calves, weanlings, or cows
The RVLs have designed a new Bovine Respiratory Disease (BRD) Package. This may change your approach to investigating group BRD problems. Follow these simple steps for better, more cost-effective diagnoses.
What sample for different age groups?
Housed calves – nasal swabs only
Calves at grass – nasal swabs, faecal samples*, Bronchoalveolar lavage (BAL) samples*
Weanlings > 6 months – nasal swabs, blood samples, faecal samples*, BAL samples*
Adult animals – nasal swabs, blood samples, faecal samples*, BAL samples*
* Required where lungworm is a differential diagnosis only
What to DO?
Nasal samples – Collect plain swabs (moistened using bottled water) from the naso-pharynx of up to six acutely affected untreated animals and place all swabs in one universal container. Nylon flocked swabs (Figure A) are the swabs of choice for PCR as they have bristles perpendicular to the shaft which improve the respiratory epithelial cell collection from the animal and also the release/recovery of the cells from the swabs at testing. Swabs with wooden shafts can inhibit the PCR assays. Charcoal swabs cannot be used for PCR testing.
As virus shedding only lasts for a few days suitable animals are those early in the course of infection i.e., have a high temperature, may not yet look depressed and nasal discharge will be serous rather than purulent. These will be pooled and tested for a range of BRD viruses (IBR, PI3, RSV, BoCo and BVD) by PCR. If needed, additional swabs MUST be taken for Mycoplasma bovis and Histophilus somni PCR.

Figure A:. Nylon flocked swab.
Blood samples - Collect serum/red top vacutainers from 10% of weanlings or adult cattle groups only (min 4-6 animals). These will be tested individually for antibodies to the common BRD viruses. As antibodies are likely to be present, paired samples (4 weeks apart) may be recommended to detect rising titres. Where cattle have been vaccinated against IBR, request gE testing and where they have not, request gB testing. If tick borne fever (TBF) is suspected as the cause of BRD, submit EDTA (purple top) tubes for TBF PCR.
Faecal samples – Where lungworm is a differential diagnosis, collect 30-50 grams of faeces from 9 animals in the affected group (first lactation cows should be targeted where adult cows are affected). Remember that clinical signs of hoose can occur in the pre patent period so a negative Baermann doesn’t rule out lungworm. This is particularly important to consider in re-infection syndrome causing coughing in adult cows.
BAL samples – Where lungworm is a differential diagnosis, collect lung lavage samples from 5-6 clinically affected or newly introduced animals (instructions on how to collect BAL samples can be found at:
These samples will be examined for evidence of lungworm or their eggs only (Figure B).
BAL samples may be useful for diagnosing lungworm infection in adult cows or other animals showing clinical signs during the pre-patent period.
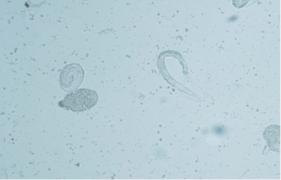
Figure B: BAL sample with larvated lungworm egg and a larva emerging from an egg.
What NOT to do?
- Don’t collect nasal swabs from less than five cattle (Reduces the likelihood of detecting an agent).
- Don’t collect swabs from chronically BRD-affected cattle (Unlikely to detect primary pathogen).
- Don’t collect nasal swabs for ‘routine bacteriology (culture or PCR)’ from any age of animal (just detects commensals).
- Don’t collect bloods from calves less than six months old (Maternally derived antibodies (MDA) still present).
- Don’t rely on carcass submissions alone to reveal causes of BRD (these often represent chronic, treated cases where the primary pathogen(s) can no longer be identified).
If in doubt about sample selection, contact the laboratory directly for advice.






